If the equilibrium constant for the reaction Ni(s)+ 4CO(g) 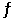 Ni(CO)4(g)is 2.72 at a certain temperature,what is the equilibrium constant for the following reaction at the same temperature?
Ni(CO)4(g)is 2.72 at a certain temperature,what is the equilibrium constant for the following reaction at the same temperature?
Ni(CO)4(g) 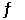 Ni(s)+ 4CO(g)
Ni(s)+ 4CO(g)
Definitions:
Learned
Pertains to knowledge or skills acquired through experience or education.
Arousal
A physiological and psychological state of being awake or reactive to stimuli.
Mental Resources
Cognitive capacities and energy available to an individual for executing tasks, processing information, and making decisions.
Kahneman
Daniel Kahneman is a psychologist known for his work on the psychology of judgment and decision-making, as well as behavioral economics, for which he was awarded the Nobel Prize in Economic Sciences.
Q12: What is the coordination number of cesium
Q18: The complex (NH<sub>3</sub>)<sub>5</sub>RuN<sub>2</sub><sup>2+</sup> can be synthesized by
Q24: The phase diagram for CO<sub>2</sub> is given
Q27: Calculate the equilibrium constant for the reaction
Q28: Calculate the [OH<font face="symbol"><sup></sup></font>] in an aqueous
Q30: The standard potential of the Cu<sup>2+</sup>/Cu electrode
Q70: The pH of a 0.0050 M aqueous
Q71: Consider the following reaction: 2Cu<sup>+</sup>(aq)<font face="symbol"></font> Cu(s)+
Q99: Graphite and diamond are two forms of
Q146: The valence electrons of elements in the