Consider the following reaction at a certain temperature: 2HI(g) 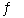 H2(g) + I2(g) K = 0.420
H2(g) + I2(g) K = 0.420
If 2.0 mol of H2(g) and I2(g) ,and 0.10 mol HI(g) were mixed in a 1.0-L flask,then
Definitions:
UPA
Uniform Partnership Act, which regulates the operation of partnerships in the absence of express agreement between the partners.
Wrongfully Withdrawing
refers to the act of improperly removing oneself or something from consideration, possession, or use without legal justification or authority.
Force Liquidation
The process of quickly selling off assets or securities, typically at lower prices, often initiated by creditors or regulatory authorities.
Criminally Liable
The legal responsibility for committing a violation of law that is designated as an offense against the state or public, subject to punishment.
Q1: The rate law for the following mechanism
Q5: For a zero-order reaction,the rate constant has
Q12: What is the end result of the
Q16: The reaction between nitrogen dioxide and carbon
Q29: The following compounds are available as 0.10
Q40: To what is the boron nitride structure
Q46: The combustion of 1 mole of ethanol,C<sub>2</sub>H<sub>5</sub>OH(l),to
Q57: Water slowly evaporates at 25<sup>o</sup>C.Which of the
Q60: The reaction CaO(s)+ H<sub>2</sub>O(l)→ Ca(OH)<sub>2</sub>(s)<br>Is spontaneous at
Q66: What is the [H<sup>+</sup>] for a solution