The following 0.1 M aqueous solutions are arranged in order of increasing pH,with the highest pH on the far right. 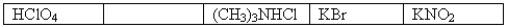 Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Which one of the following 0.10 M aqueous solutions should be placed in the empty box?
Definitions:
Q31: Calculate the equilibrium constant for the reaction
Q44: Predict the sign (+,<font face="symbol"></font>,0)of the entropy
Q44: For a solution labeled "0.10 M H<sub>3</sub>PO<sub>4</sub>(aq),"<br>A)[H<sub>2</sub>PO<sub>4</sub><font
Q46: What is the relationship between K and
Q49: Consider the reaction NOBr(g)<font face="symbol"></font> NO(g)+ ½Br<sub>2</sub>(g)<br>A
Q66: What is the [H<sup>+</sup>] for a solution
Q81: For the reaction 2SO<sub>3</sub>(g)<font face="symbol"></font> 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)<br><font
Q83: Calculate the lattice enthalpy of potassium chloride
Q92: In the cell shown above,A is a
Q94: True or false: the boiling point of