Calculate the value of the equilibrium constant for the reaction AgCl(s) + 2NH3(aq) 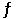 Ag(NH3) 2+(aq) + Cl(aq)
Ag(NH3) 2+(aq) + Cl(aq)
Given Ksp = 1.6 1010 for silver chloride and Kf = 1.6 107 for the ammonia complex of Ag+ ions,Ag(NH3) 2+.
Definitions:
Dramaturgy
A sociological perspective that interprets social interactions as theater, where individuals take on roles and act them out for a social audience.
Dramaturgical Concepts
Refers to ideas stemming from the theory which views social interactions as performances, where individuals are actors on metaphorical stages.
Backstage
A term from sociology, specifically Erving Goffman's dramaturgical analysis, referring to private areas of our lives where we prepare for and recover from the public "performance" of our social roles.
Symbolic Interaction
Refers to the process by which individuals use symbols and language to interact and communicate, creating shared meanings.
Q7: What is the shape of XeOF<sub>4</sub>?<br>A)trigonal bipyramidal<br>B)square
Q33: Calculate <font face="symbol"></font>G at 298 K for
Q36: Which of the following complexes is (are)chiral?
Q63: The galvanic cell shown above uses the
Q77: The lattice enthalpy of calcium oxide is
Q84: Calculate the normal boiling point of chloroform,given
Q91: The osmotic pressure of 1.00 g of
Q92: Which of the following would have the
Q146: The valence electrons of elements in the
Q147: Consider the reaction Fe(ClO<sub>4</sub>)<sub>3</sub>(aq)+ 6H<sub>2</sub>O(l)<font face="symbol"></font> Fe(H<sub>2</sub>O)<sub>6</sub><sup>3+</sup>