Use the following to answer questions 55-58: 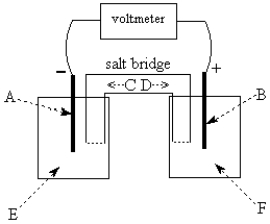
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
Definitions:
Q19: All of the oxides of the following
Q32: The fractional composition diagram for the amino
Q33: Which of the following pairs have a
Q36: When a <font face="symbol"></font> particle is emitted,the
Q40: All of the following form clathrates with
Q43: Consider the reaction<br>2SO<sub>2</sub>(g)+ O<sub>2</sub>(g)<font face="symbol"></font> 2SO<sub>3</sub>(g)<br>At equilibrium
Q44: What type of particle is emitted in
Q76: The phase diagram for sulfur is given
Q84: Estimate the enthalpy of vaporization of water
Q89: If AgNO<sub>3</sub>(aq)is added to a 1 M