The reaction 2NO(g) + O2(g) 2NO2(g) has Hr = 114 kJmol1.A possible mechanism for this reaction is 2NO(g) 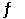 N2O2(g)
N2O2(g)
Rapid equilibrium,K
N2O2(g) + O2(g) 2NO2(g)
Slow,k
If the activation energy for the reverse reaction is 225 kJmol1,what is the activation energy for the forward reaction?
Definitions:
Cash Equivalents
Short-term, highly liquid investments that are readily convertible to known amounts of cash and close to their maturity.
Basis of Measurement
The specific basis chosen to measure various types of assets, liabilities, income, and expenses, such as historical cost, fair value, and amortized cost.
Accrual
An accounting method that records revenues and expenses when they are incurred, regardless of when cash transactions occur.
Net Cash Inflow
The difference between all cash received and all cash spent by a company during a specific period.
Q17: What nuclide undergoes <font face="symbol"></font> decay to
Q27: Using the cell shown above,A is a
Q30: Consider the complex trans-[Co(NH<sub>3</sub>)<sub>4</sub>Cl<sub>2</sub>]Cl.Draw the structure of
Q36: The conjugate base of OH<font face="symbol"><sup></sup></font> is<br>A)H<sup>+</sup>.<br>B)OH<font
Q52: The complex [Ru(OH<sub>2</sub>)<sub>3</sub>Cl<sub>3</sub>]<br>A)is chiral.<br>B)has 2 geometric isomers,
Q53: What is the product of benzoic acid
Q54: Consider the reaction 2N<sub>2</sub>O(g)<font face="symbol"></font> 2N<sub>2</sub>(g)+ O<sub>2</sub>(g)<br>Rate
Q63: Of the following,which is not a Lewis
Q65: Autoprotolysis occurs to a much smaller extent
Q91: Which of the following undergo nitration more