Use the following to answer questions 55-58: 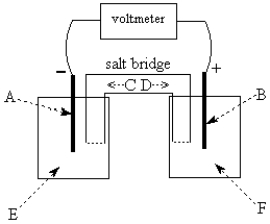
-The galvanic cell shown above uses the half-cells Mg2+/Mg and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
Definitions:
Japan
A country located in East Asia, known for its rich culture, technological advancements, and economic significance.
Bond
A fixed income instrument that represents a loan made by an investor to a borrower, usually corporations or governmental agencies, which includes the terms of the loan, interest payments, and the time at which the loaned funds must be repaid.
Interest Rate Parity
An economic theory stating that the difference in interest rates between two countries is equal to the expected change in exchange rates between their currencies.
Unbiased Forward Rates
Financial theory suggesting that forward exchange rates should be an unbiased predictor of future spot exchange rates.
Q15: The rate law for a reaction can
Q48: Consider the following compounds and their
Q63: The standard potential of the Cu<sup>2+</sup>/Cu electrode
Q65: Calculate the number of moles of
Q69: Both Cu<sup>2+</sup>(aq)and Zn<sup>2+</sup>(aq)are colorless.
Q80: The equilibrium constant expression for the
Q89: What is the molarity of OH<sup>-</sup> in
Q110: If the value of K<sub>b</sub> for
Q131: Diborane has<br>A) 2 bridging hydrogens and 4
Q185: Aluminum oxide dissolves in aqueous base .What