Use the following to answer questions 55-58: 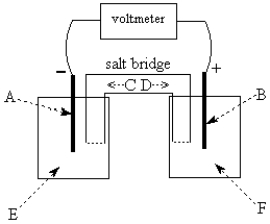
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
Definitions:
Urination
The process of excreting urine from the urinary bladder through the urethra to the outside of the body, an essential function for eliminating waste.
Filtration
A process that separates solids from liquids or gases using a filter medium that only allows the fluid to pass through.
Reabsorption
The process by which substances are taken back into the bloodstream from the fluids in the kidneys.
Malpighian Tubules
Excretory structures in insects and some other arthropods that remove wastes from the body and help in osmoregulation.
Q9: For the proposed mechanism<br>A + X
Q24: When <sup>214</sup>Pb decays as part of
Q40: The valence electrons of elements in the
Q60: If the standard potential for Ti<sup>3+</sup>(aq)/Ti<sup>2+</sup>(aq)
Q64: The pH of a 0.0050 M aqueous
Q66: How many oxidation states do most d-block
Q82: The amino acid alanine, HOOC-CH(CH<sub>3</sub>)NH<sub>3</sub><sup>+</sup>,Has K<sub>a1</sub>
Q162: The formula of phosphorus(V) oxide is<br>A) P<sub>2</sub>O<sub>4</sub>.<br>B)
Q191: All the following compounds become less stable
Q282: Given:<br>Ag<sup>+</sup>(aq)+ e<sup>-</sup> <span class="ql-formula" data-value="\rightarrow"><span