Use the following to answer questions 55-58: 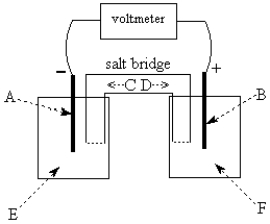
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Theelectrode B could be inert platinum metal or lead.
Definitions:
Tough Question
One that makes a person or group stop and think about why they are doing or not doing something.
Group Reflection
The process by which a group collectively thinks about and discusses its past actions, decisions, and outcomes to learn and improve.
Deserve Punishment
The belief that individuals who have committed wrongdoing or broken rules should face consequences for their actions.
Consideration
The degree to which the leader creates an environment of emotional support, warmth, friendliness, and trust.
Q8: Write the formula of the hydride ion.
Q26: The metalloproteins hemoglobin and vitamin B<sub>12</sub> contain
Q36: Calculate the equilibrium constant for the reaction
Q58: Use tabulated thermodynamic data to estimate the
Q63: If the average rate of formation
Q67: Bromine can be obtained from brines by
Q116: Which of the following is the strongest
Q125: The alkali metals all react with water
Q147: Both H<sub>2</sub>O and H<sub>2</sub>S can participate in
Q162: The formula of phosphorus(V) oxide is<br>A) P<sub>2</sub>O<sub>4</sub>.<br>B)