Use the following to answer questions 55-58: 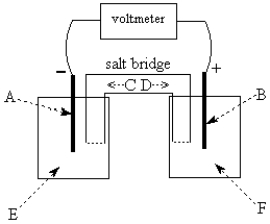
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn,and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.The electrode B could be inert platinum metal or zinc.
Definitions:
First-In, First-Out
An inventory valuation method that assumes goods are sold in the order they are acquired, with the cost of the oldest products being used to calculate cost of goods sold first.
Conversion
The process of changing assets, investments, or data from one form to another, often referring to the conversion of currencies or converting raw materials into finished goods.
Equivalent Units Of Production
The number of production units that could have been completed within a given accounting period, given the resources consumed.
First-In, First-Out
An inventory accounting method where goods first purchased or manufactured are the first ones to be sold.
Q1: Calculate the concentration of argon in lake
Q18: An endothermic reaction is most likely
Q21: Which of the following complexes is chiral?<br>A)
Q53: The nuclear equation for the disintegration
Q56: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math
Q71: For the reaction<br>2CaSO<sub>4</sub>(s)<br> <span class="ql-formula" data-value="f"><span
Q99: Which of the following is diamagnetic?<br>A) O<sub>2</sub><sup>2</sup><sup>-</sup><br>B)
Q140: Consider the titration of 15.0 mL of
Q182: An example of a spontaneous process
Q193: Which of the following mixtures gives a