Use the following diagram of a cell to answer questions 59-64: 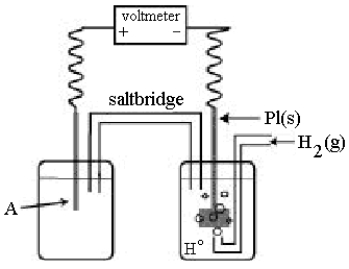
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE) .If the voltmeter reading is -0.76 V,
Which half-reaction occurs in the left-hand cell compartment?
Definitions:
Order Filling
The process involved in gathering and packaging goods or materials to fulfil customer orders.
First Stage Allocations
The initial step in activity-based costing where overhead costs are allocated to various activities.
Overhead Cost
General operating costs not directly tied to a specific product or service, including utilities, rent, and administrative salaries.
Order Filling
The process of completing customer orders, including picking, packing, and shipping the requested items.
Q14: Consider the reaction below.<br>2A + B<sub>2</sub>
Q20: Elements with an odd number of
Q24: For a 0.10 M solution of
Q44: The standard potential of the cell<br>Ag(s)|AgCl(s)MCl<sup>-</sup>(aq|Cu<sup>2+</sup>(aq)|Cu(s)<br>Is
Q47: Write the charge balance equation for a
Q67: The phase diagram for CO<sub>2</sub> is given
Q68: Calculate the vapor pressure of ethyl alcohol,
Q77: The half-life of plutonium-239 is 24 100
Q80: Sodium is produced by electrolysis of molten
Q191: If the K<sub>sp</sub> of AgI is 1.5