Use the following to answer questions 55-58: 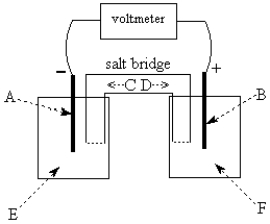
-The galvanic cell shown above uses the half-cells Mg2+/Mg and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
Definitions:
Marginal Benefits
The additional enjoyment or advantage obtained from using one more unit of a good or service.
Pollution Emissions
The release of pollutants, harmful chemicals, or substances into the environment, typically as a byproduct of industrial activity or transportation.
Total Pollution Emissions
Total Pollution Emissions refer to the sum of all harmful pollutants released into the environment by human activities, including industrial processes, vehicles, and agriculture.
Marginal Benefits
The additional satisfaction or utility gained from consuming or producing one more unit of a good or service.
Q20: For the complex ion [Co(NCO)<sub>2</sub>(PH<sub>3</sub>)<sub>4</sub>]<sup>+</sup>, how many
Q22: What is the shape of Pt(NH<sub>3</sub>)<sub>2</sub>Cl<sub>2</sub> assuming
Q68: What is the name of the complex
Q77: Al<sub>2</sub>O<sub>3</sub>(s) dissolves in aqueous acid to produce<br>A)
Q80: State whether each of the following oxides
Q102: Which of the following statements about ion
Q159: When pyridinium chloride is added to C<sub>5</sub>H<sub>5</sub>N(aq),<br>A)
Q162: The galvanic cell shown above uses the
Q182: An example of a spontaneous process
Q260: All of the following are Lewis bases