Use the following to answer questions 55-58: 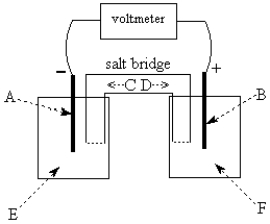
-The galvanic cell shown above uses the half-cells Pb2+/Pb and Zn2+/Zn, and a salt bridge containing KCl(aq).The voltmeter gives a positive voltage reading.Identify A and write the half-reaction that occurs in that compartment.Does the size of the electrode A increase or decrease during operation of the cell? What is the voltmeter reading?
Definitions:
Real Income
The income of an individual or group after adjusting for inflation, reflecting the true purchasing power of the money earned.
Working-Class Americans
Individuals in the United States who are employed in manual labor or industrial jobs, often with lower wages and less job security.
Profound Recession
A term indicating a severe and prolonged economic downturn, deeper than a standard recession, with significant impacts on employment and economic growth.
NAFTA
The North American Free Trade Agreement, a treaty entered into by the United States, Canada, and Mexico to eliminate trade barriers and facilitate the exchange of goods and services.
Q7: In the reaction<br>Cu<sup>2+</sup>(aq)+ 4NH<sub>3</sub>(aq) <span class="ql-formula"
Q68: Which of the following are all allotropes?<br>A)
Q73: Which of the following has the lowest
Q78: The atomic radii of the Period 6
Q87: How many unpaired electrons are expected for
Q89: What is the molarity of OH<sup>-</sup> in
Q92: Calculate the standard entropy for the
Q166: Lithium and sodium burn in air to
Q172: Quicklime, CaO(s),Reacts with C(s)To produce A plus
Q249: For HF, pK<sub>a</sub> = 3.45.What is the