Use the following diagram of a cell to answer questions 59-64: 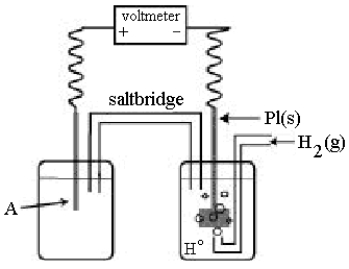
-In the cell shown above, A is a standard Zn2+/Zn electrode connected to a standard hydrogen electrode (SHE) .If the voltmeter reading is -0.76 V,
Which half-reaction occurs in the left-hand cell compartment?
Definitions:
Neglect
The failure to provide for the basic needs of someone who cannot care for themselves, resulting in significant harm or risk of harm.
Hand-Mouth Coordination
The ability to move the hands and fingers in a precise manner in coordination with the eyes, often relevant in feeding and object manipulation.
Visual Acuity 20/40
A measure of eyesight indicating that a person can see at 20 feet what a person with normal vision can see at 40 feet.
Internal Abdominal Pain
Pain originating within the abdominal cavity, potentially indicative of various health conditions or diseases.
Q1: The maximum oxidation states for chromium and
Q9: The nuclide <sup>7</sup>Be decays by electron capture.
Q38: For a solution labeled "0.10 M H<sub>3</sub>PO<sub>4</sub>(aq),"<br>A)
Q63: The standard potential of the Cu<sup>2+</sup>/Cu electrode
Q105: Consider the following cell:<br>Zn(s)|Zn<sup>2+</sup>(aq,0.10 M)m Cu<sup>2+</sup>(aq,0.10 M)|Cu(s)<br>At
Q121: Which of the following has the largest
Q144: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q167: What is the pH of an aqueous
Q218: Calculate the [OH<sup>-</sup>] in an aqueous solution
Q252: When a lead-acid battery discharges,sulfuric acid is