Use the following diagram of a cell to answer questions 59-64: 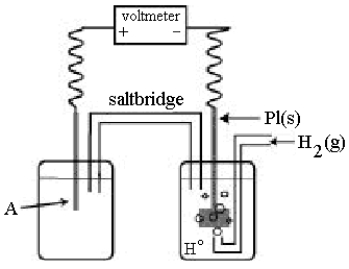
-In the cell shown above,A is a standard Ag+/Ag electrode connected to a standard hydrogen electrode (SHE).If the voltmeter reading is +0.80 V,which electrode is negative?
Definitions:
Budget
An itemized forecast of an entity’s income and expenses expected for a specific period, usually showing how much money it intends to earn, spend, and save.
Donations
Voluntary transfers of resources, such as money or goods, to individuals or organizations without the expectation of direct return or compensation.
Spending Variances
Differences between the budgeted or standard cost amounts and the actual costs incurred.
Favorable
A term used in financial analysis to describe a situation, condition, or variance that leads to a positive outcome or better-than-expected results.
Q32: In the Michaelis-Menten mechanism of enzyme reaction,
Q34: Sulfur dioxide can act as both an
Q37: Sodium borohydride is an oxidizing agent.
Q72: the van't Hoff i of HBr,HCl,and HF
Q82: What is the element produced when <sup>83</sup>Sr
Q91: For the reaction 2NH<sub>3</sub>(g) <span class="ql-formula"
Q120: The standard potential of the Cu<sup>2+</sup>/Cu electrode
Q129: The reaction CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub>(g) <span class="ql-formula" data-value="\rightarrow"><span
Q147: Both H<sub>2</sub>O and H<sub>2</sub>S can participate in
Q170: For the reaction<br>NH<sub>3</sub>(g)+ H<sub>2</sub>S(g) <span