How many moles of magnesium oxide are produced by the reaction of 1.82 g of magnesium nitride with 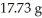 of water?
of water?
Mg3N2 + 3H2O → 2NH3 + 3MgO
Definitions:
Evolutionary Change
The process through which species undergo changes over time due to genetic variations and natural selection.
New Mutations
Genetic alterations that occur for the first time in a DNA sequence, contributing to genetic variation and potentially to evolution.
Fitness
The genetic contribution of an individual to subsequent generations, often measured by the individual's reproductive success compared to others in the population.
Influenza Virus
A highly contagious viral infection that attacks the respiratory system, causing symptoms like fever, cough, and aches.
Q4: The formula of the chromate ion is
Q31: What is the maximum mass in grams
Q32: If a iron atom loses 2 electrons
Q37: How many different principal quantum numbers can
Q65: The correct name for SO is _.<br>A)sulfur
Q86: The element _ is the most similar
Q94: The spectator ions in the reaction between
Q114: The balanced reaction between aqueous potassium hydroxide
Q142: _ is the abbreviation for the prefix
Q176: A sample of CH<sub>2</sub>F<sub>2</sub> with a mass