How many moles of magnesium oxide are produced by the reaction of 1.82 g of magnesium nitride with 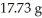 of water?
of water?
Mg3N2 + 3H2O → 2NH3 + 3MgO
Definitions:
Double-Declining Balance
A method of accelerated depreciation where an asset’s book value is decreased at double the rate of its straight-line depreciation.
Straight-Line
A method of calculating depreciation or amortization by dividing the cost of an asset equally over its useful life.
Double-Declining Balance
A method of accelerated depreciation that doubles the normal depreciation rate, reducing the value of an asset more quickly in its early years.
Salvage Value
The projected value of an asset when it reaches the end of its functional lifespan.
Q10: How many moles of pyridine (C<sub>5</sub>H<sub>5</sub>N)are contained
Q11: How many moles of sodium carbonate contain
Q11: How many grams of H<sub>3</sub>PO<sub>4</sub> are in
Q16: Hydrogen gas and bromine gas react to
Q26: What is the mass % of carbon
Q32: The formula weight of Zn(ClO<sub>4</sub>)<sub>2</sub> is _
Q62: A 5.00-g sample of copper metal at
Q81: How many grams of NaOH (MW =
Q144: What is the frequency (s<sup>-1</sup>)of electromagnetic radiation
Q180: Of the following,the smallest and lightest subatomic