A titration reached the equivalence point when 16.1 mL of 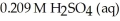 was added to
was added to 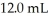 of NaOH (aq) of unknown concentration.What is the concentration (M) of this unknown NaOH solution?
of NaOH (aq) of unknown concentration.What is the concentration (M) of this unknown NaOH solution?
Definitions:
Single Covalent
A type of chemical bond where two atoms share one pair of electrons.
Ionic Bond
A chemical bond formed between two ions with opposite charges through the transfer of electrons from one atom to another.
Triple Covalent
A type of chemical bond where three pairs of electrons are shared between two atoms.
Hydrogen Bond
An unstable linkage between two molecules due to the electrostatic force acting between a proton within one molecule and an electronegative atom in the other.
Q43: Which of these metals will be oxidized
Q50: The temperature of a 15-g sample of
Q58: Of the species below,only _ is not
Q59: A 22.5-g sample of ammonium carbonate contains
Q64: The formula for chromium (II)iodide is CrI<sub>2</sub>.
Q68: Calculate the concentration (M)of sodium ions in
Q82: Which one of the following is a
Q92: Of the following transitions in the Bohr
Q138: Which of the following correctly represents the
Q162: The de Broglie wavelength of a 0.02900