What volume (mL) of a concentrated solution of sodium hydroxide (6.00 M) must be diluted to 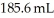 to make a
to make a 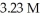 solution of sodium hydroxide?
solution of sodium hydroxide?
Definitions:
Double-Declining-Balance Method
An accelerated method of depreciation that doubles the normal depreciation rate.
Journal Entry
A record of a transaction in the accounting journal that may affect one or more accounts.
Residual Value
The projected worth of an asset at the time of sale, after it has served its purpose.
Accumulated Depreciation
The cumulative depreciation of an asset up to a single point in its life, representing the wear and tear, deterioration, or obsolescence of the asset.
Q26: What is the energy of a photon
Q27: Which one of the following polyatomic ions
Q41: All of the following are considered fossil
Q92: Of the following transitions in the Bohr
Q94: How many electrons does the Al<sup>3+</sup> ion
Q98: Vertical columns of the periodic table are
Q102: The formula weight of silver chromate (Ag<sub>2</sub>CrO<sub>4</sub>)is
Q139: All of the following noble gases have
Q157: The element carbon exists in several forms
Q159: What is the concentration of nitrate ions