The value of ΔH° for the reaction below is -6535 kJ. ________ kJ of heat are released in the combustion of 16.0 g of 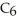
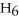 (l) ?
(l) ?
2C6H6 (l) + 15O2 (g) → 12CO2 (g) + 6H2O (l)
Definitions:
Social Mechanisms
Processes and structures within society that contribute to particular social outcomes, influencing how societal norms and interactions occur.
Gender Distinctions
Socially and culturally constructed differences between males and females, often based on attributes, roles, and expectations.
Interactional
Pertaining to or involving interaction between individuals or entities.
Societal
Pertaining to or involving society and its institutions, norms, and values.
Q27: HNO<sub>2</sub> is a strong acid.
Q53: Given the data in the table below,ΔH°<sub>rxn</sub>
Q63: _ is an explosive made of nitroglycerine
Q114: Which of the following statements is not
Q120: Which of the following is an oxidation-reduction
Q126: The wavelength of light emitted from a
Q133: The most abundant fossil fuel is _.<br>A)natural
Q137: The formula weight of PbCO<sub>3</sub> is _
Q147: What is the concentration of chloride ions
Q147: Calculate the kinetic energy in J of