The value of ΔH° for the reaction below is -1107 kJ: 2Ba (s) + O2 (g) → 2BaO (s)
How many kJ of heat are released when 5.75 g of Ba (s) reacts completely with oxygen to form 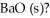
Definitions:
Inferiority
A feeling of being lower in status or quality compared to others, which can impact an individual's self-esteem and social interactions.
Thin Slices
Rapid assessments or judgments made based on a very brief exposure to a slice of behavioral or situational information.
Stereotypes
Oversimplified generalized images or ideas held about a group of people, leading to categorization based on those perceptions rather than individual characteristics.
Personal Attributions
The process of ascribing causes to one's own behavior and outcomes, highlighting the influence of internal factors.
Q3: Which electron configuration represents a violation of
Q27: The value of ΔH° for the reaction
Q69: Dynamite consists of nitroglycerine mixed with diatomaceous
Q86: All of the following statements are true
Q92: The process by which metal in the
Q106: The net ionic equation for the reaction
Q146: The ground-state configuration of fluorine is _.<br>A)[He]2s<sup>2</sup>2p<sup>2</sup><br>B)[He]2s<sup>2</sup>2p<sup>3</sup><br>C)[He]2s<sup>2</sup>2p<sup>4</sup><br>D)[He]2s<sup>2</sup>2p<sup>5</sup><br>E)[He]2s<sup>2</sup>2p<sup>6</sup>
Q153: A line spectrum contains radiation of _
Q157: Sulfur and oxygen react to produce sulfur
Q161: What volume (mL)of a concentrated solution of