Given the following reactions N2 (g) + O2 (g) → 2NO (g) ΔH = +180.7 kJ
2NO (g) + O2(g) → 2N 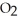 (g) ΔH = -113.1 kJ
(g) ΔH = -113.1 kJ
The enthalpy of reaction for
4NO (g) → 2NO2 (g) + N2 (g)
Is ________ kJ.
Definitions:
Pink Collar Jobs
Employment sectors traditionally dominated by women, such as nursing, teaching, and clerical work, characterized by lower pay and less prestige than male-dominated fields.
Patriarchal Society
A social system in which men hold primary power and predominate in roles of political leadership, moral authority, social privilege, and control of property.
Caretaking
The act of providing care for someone, including looking after their well-being, health, and the maintenance of their living environment.
Prestige
The social honor people are given because of their membership in well-regarded social groups.
Q20: Electromagnetic radiation with a wavelength of 641
Q21: The effective nuclear charge of an atom
Q27: Which noble gas has the highest first
Q48: A 750 nm wavelength light corresponds to
Q67: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q78: The molarity of a solution prepared by
Q114: When the following equation is balanced,the coefficient
Q117: The element that has a valence configuration
Q125: How many grams of NaOH (MW =
Q144: [He]2s<sup>2</sup>2p<sup>5</sup> is the electron configuration for _.