The value of ΔH° for the following reaction is -3351 kJ: 2Al (s) + 3O2(g) → 2Al2O3(s)
The value of ΔH°f for Al2
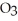 (s) is ________ kJ.
(s) is ________ kJ.
Definitions:
Cognitive Skills
Mental abilities involved in the process of acquiring knowledge and understanding through thought, experience, and the senses, such as perception, reasoning, memory, and attention.
Treatment Expense
The cost associated with medical care, including diagnostics, therapy, medication, and other interventions necessary to treat or manage conditions.
Adolescent Suicide Rate
The statistical measure of the number of suicide deaths in a given population of adolescents, usually per 100,000 individuals per year.
Antidepressants
Medications designed to improve symptoms of depression by altering chemical activity in the brain.
Q15: An electron cannot have the quantum numbers
Q21: Calculate the percentage by mass of oxygen
Q30: _ has been shown to form compounds
Q87: Peroxide or superoxides can be formed with
Q112: How many sulfur dioxide molecules are there
Q128: Ozone is a a(n)_ of oxygen.<br>A)isotope<br>B)allotrope<br>C)precursor<br>D)peroxide<br>E)free radical
Q134: The compound NH<sub>4</sub>Cl is a weak acid.
Q141: Which of the following correctly represents the
Q146: CH<sub>3</sub>OH (l)decomposes into carbon monoxide and hydrogen
Q161: What volume (mL)of a concentrated solution of