Given the data in the table below and ΔH°rxn for the reaction SO2Cl2 (g) + 2H2O (l) → H2SO4 (l) + 2HCl (g) ΔH° = -62 kJ
ΔH°f of HCl (g) is ________ kJ/mol. 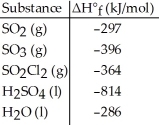
Definitions:
Motivational Interviewing
A counseling approach that aids in eliciting behavior change by helping clients to explore and resolve ambivalence.
Transtheoretical Model
A theory of behavior change that assesses an individual's readiness to act on a new healthier behavior, and provides strategies to guide the individual through the stages of change.
Precontemplation
A stage in the Transtheoretical Model of Behavior Change where the individual is not yet considering change or unaware of the need to change.
Action
The process of doing something in order to achieve a specific goal or result.
Q15: The specific heat capacity of methane gas
Q21: Pure acetic acid (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)is a liquid and
Q32: The Lewis structure of PF<sub>3</sub> shows that
Q36: Of the reactions below,which one is a
Q41: As the number of covalent bonds between
Q71: There are _ oxygen atoms in 30
Q78: The molarity of a solution prepared by
Q96: Of the bonds C-C,C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="Of
Q159: What is the concentration of nitrate ions
Q161: Which of the groups in the periodic