The temperature of a 35.1 g sample of iron increases from 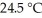 to
to 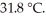 If the specific heat of iron is
If the specific heat of iron is 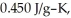 how many joules of heat are absorbed?
how many joules of heat are absorbed?
Definitions:
Conserve
To use resources carefully and efficiently to avoid waste and ensure sustainability for future generations.
Future Value
The value of a current asset at a specified date in the future based on an assumed rate of growth over time.
Water Resource Economists
Specialists who analyze the use, management, and conservation of water resources through the lens of economic theories and models.
Specific Places
This term doesn't align directly with a specific economic concept; it could relate to locations significant for economic activities or market transactions.
Q8: For the following reactions,the ΔH°<sub>rxn</sub> is NOT
Q54: A solution of CH<sub>3</sub>F is prepared by
Q64: The balanced reaction between aqueous nitric acid
Q67: In which reaction does the oxidation number
Q118: When dissolved in water,_ produces a basic
Q132: Consider the general valence electron configuration of
Q137: The formula weight of PbCO<sub>3</sub> is _
Q152: A compound that is composed of only
Q163: Which of the following correctly represents the
Q172: _ is unique among the elements because