The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l) → CO (g) + 2H2 (g)
How many kJ of heat are consumed when 15.5 g of C 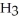 OH (l) decomposes as shown in the equation?
OH (l) decomposes as shown in the equation?
Definitions:
Filum Terminale
A slender filament of connective tissue that extends from the bottom of the spinal cord and anchors it within the vertebral column.
Conus Medullaris
The tapered, lower end of the spinal cord, found at the level of the first and second lumbar vertebrae.
Cerebrum
The largest part of the brain, responsible for voluntary activities, intelligence, memory, and personality.
Sulcus
A groove or furrow on the surface of an organ, often referring to the brain, indicating a division between lobes or gyri.
Q49: In which species does nitrogen have an
Q104: What are the respective concentrations (M)of Mg<sup>2+</sup>
Q105: Given the following reactions N<sub>2</sub> (g)+ O<sub>2</sub>
Q105: A 13 kg object is traveling at
Q111: There are _ protons,_ electrons,and _ neutrons
Q128: How many electrons are in the Lewis
Q165: Which solution has the same number of
Q172: _ is unique among the elements because
Q177: The formula weight of a substance is
Q181: The wavelength of light that has a