Multiple Choice
The value of ΔH° for the reaction below is -1107 kJ: 2Ba (s) + O2 (g) → 2BaO (s)
How many kJ of heat are released when 5.75 g of Ba (s) reacts completely with oxygen to form 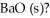
Definitions:
Related Questions
Q8: A titration reached the equivalence point when
Q22: Which of the following 0.300 M solutions
Q30: _ has been shown to form compounds
Q34: An alkaline earth metal forms a compound
Q63: Which one of the following compounds is
Q82: Based on the equations below,which metal is
Q91: The lowest energy shell that contains f
Q93: According to the Heisenberg Uncertainty Principle,it is
Q98: Based on the octet rule,phosphorus most likely
Q160: Oxides of the active metals combine with