Given the following reactions N2 (g) + O2 (g) → 2NO (g) ΔH = +180.7 kJ
2NO (g) + O2(g) → 2N 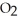 (g) ΔH = -113.1 kJ
(g) ΔH = -113.1 kJ
The enthalpy of reaction for
4NO (g) → 2NO2 (g) + N2 (g)
Is ________ kJ.
Definitions:
Commitment To Values
A dedication or pledge to uphold and act according to specific principles or standards of behavior.
Pragmatic Incrementalism
A strategy that involves making continuous, small-scale improvements or changes in a practical and realistic manner to achieve a larger goal.
Serving The Public
The act or process of providing services, making decisions, or conducting work that benefits or is intended to benefit the general public.
Relative Calm
A state of peace or tranquility compared to a previous or typical level of activity or agitation.
Q7: What volume (mL)of 7.48 × 10<sup>-2</sup> M
Q8: _ is isoelectronic with krypton.<br>A)Se<sup>2-</sup><br>B)Se<sup>3-</sup><br>C)Br<br>D)Se<sup>2+</sup><br>E)Te<sup>2-</sup>
Q13: Which of the following metals differ in
Q39: The energy of a photon that has
Q51: The central atom in _ does not
Q58: Of the species below,only _ is not
Q105: Of the possible bonds between carbon atoms
Q113: Which of the following has the largest
Q135: Lithium and nitrogen react in a combination
Q137: All of the following reactions concerning alkali