The value of ΔH° for the following reaction is -3351 kJ: 2Al (s) + 3O2(g) → 2Al2O3(s)
The value of ΔH°f for Al2
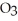 (s) is ________ kJ.
(s) is ________ kJ.
Definitions:
Product Pricing
The strategy of setting the appropriate cost for a product based on production costs, competition, target audience affordability, and market conditions.
Retain Customer
Strategies and activities aimed at keeping a company's existing customers engaged and satisfied with its products or services, thereby increasing loyalty and reducing churn.
Business Plan
A description of the direction for a new business and the financing needed to operate it.
Comprehensive
Inclusive, covering a wide scope or containing a great deal of information.
Q17: A group of ions all containing the
Q33: What is the de Broglie wavelength (m)of
Q55: The formula of a salt is XC
Q55: How many joules of heat are absorbed
Q80: Given the following reactions 2NO → N<sub>2</sub>
Q91: At what velocity (m/s)must a 417.3 g
Q146: The ground-state configuration of fluorine is _.<br>A)[He]2s<sup>2</sup>2p<sup>2</sup><br>B)[He]2s<sup>2</sup>2p<sup>3</sup><br>C)[He]2s<sup>2</sup>2p<sup>4</sup><br>D)[He]2s<sup>2</sup>2p<sup>5</sup><br>E)[He]2s<sup>2</sup>2p<sup>6</sup>
Q161: What volume (mL)of a concentrated solution of
Q163: What is the mass in grams of
Q173: What volume (mL)of a 5.45 M lead