Given the data in the table below,ΔH°rxn for the reaction C2H5OH (l) + O2 (g) → CH3CO2H (l) + H2O (l)
Is ________ kJ. 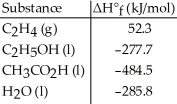
Definitions:
Improved Oven
A term referring to an oven with enhanced features or efficiency, signifying technological innovation or investment in capital goods.
Bakers
Professionals who prepare and bake breads, pastries, and other baked goods.
Steamed Milk
Milk that has been heated and aerated through the introduction of steam, often used in coffee beverages.
Equilibrium Quantity
The quantity of goods or services supplied that is exactly equal to the quantity demanded at the market equilibrium price.
Q11: What is the coefficient of Na when
Q39: The primary component of natural gas is
Q48: The first ionization energies of the elements
Q54: A 26.9 g rock rolls down the
Q55: How many joules of heat are absorbed
Q67: _ is credited with developing the concept
Q94: What is the frequency (s<sup>-1</sup>)of a photon
Q110: Of the following transitions in the Bohr
Q126: Which of the following Lewis structures would
Q141: When _ is constant,the enthalpy change of