The value of ΔH° for the following reaction is 177.8 kJ.The value of Δ 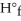 for CaO(s) is ________ kJ/mol. CaCO3 (s) → CaO (s) + CO2 (g)
for CaO(s) is ________ kJ/mol. CaCO3 (s) → CaO (s) + CO2 (g) 
Definitions:
Context Effect
The influence of environmental factors on one's perception, performance, and decision-making.
Constructed Questions
Questions designed to require a constructed response or answer, such as those requiring written or elaborated upon replies.
Double-Barreled
A question or statement that attempts to address more than one issue, but only allows for one answer, potentially causing confusion or inaccurate responses.
Loaded Question
A question that contains a controversial or unjustified assumption, making it difficult to answer without implicating oneself in some undesirable way.
Q7: How many grams of hydrogen are in
Q15: The specific heat capacity of methane gas
Q23: What is the concentration (M)of CH<sub>3</sub>OH in
Q53: A 3.82-g sample of magnesium nitride is
Q55: The de Broglie wavelength of a _
Q73: Which of the following correctly lists the
Q73: High energy and low wavelength light has
Q96: What is the concentration (M)of CH<sub>3</sub>OH in
Q97: An electron transition from n = 2
Q146: CH<sub>3</sub>OH (l)decomposes into carbon monoxide and hydrogen