Given the data in the table below,ΔH°rxn for the reaction Ag2O (s) + H2S (g) → Ag2S (s) + H2O (l)
Is ________ kJ. 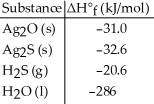
Definitions:
City Historians
Individuals specializing in the history of specific cities, often focusing on urban development, culture, and significant events.
Past Participle
A grammatical form used to indicate a completed action or condition, typically ending in -ed, -d, or -n in English.
Guinness Book
A record book that documents world records of human achievements as well as extreme natural events.
Ripley's Believe-It-or-Not
A franchise comprising books, a television show, and museums, showcasing odd, unusual, or unbelievable facts and items.
Q14: How many moles of Cu<sup>2+</sup> ions are
Q35: The ΔH<sub>vap </sub>of water is 40.7 kJ
Q56: Which two elements have the same ground-state
Q83: If an electron has a principal quantum
Q114: At maximum,an d-subshell can hold _ electrons.<br>A)10<br>B)6<br>C)2<br>D)8<br>E)14
Q122: The spectator ions in the reaction between
Q131: Calculate the percentage by mass of hydrogen
Q153: What are the respective concentrations (M)of K<sup>+</sup>
Q160: Which electron configuration represents a violation of
Q184: Which group is represented by a ns<sup>2</sup>np<sup>1</sup>