Given the data in the table below,ΔH°rxn for the reaction 3Cl2 (g) + PH3 (g) → PCl3 (g) + 3HCl (g)
Is ________ kJ. 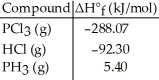
Definitions:
Inventory Decisions
The process of determining the optimal level and timing of inventory to minimize costs and meet demand.
Aluminum Manufacturer
A company specialized in producing aluminum through the processing of raw materials, often involving the bauxite ore.
Lobbying Agency
An organization that advocates on behalf of a group or individual to influence public policy and decisions.
Value-Chain Analysis
Value-Chain Analysis involves examining the steps involved in producing a product or providing a service to optimize the value delivered to customers and increase efficiency.
Q1: What color of visible light has the
Q17: Which one of the following is considered
Q45: An electron cannot have the quantum numbers
Q45: Which equation correctly represents the electron affinity
Q72: The kinetic energy of a 23.2-g object
Q117: List seven nonmetals that exist as diatomic
Q127: A certain alcohol contains only three elements,carbon,hydrogen,and
Q133: The ion ICl<sub>4</sub><sup>-</sup> has _ valence electrons.<br>A)34<br>B)35<br>C)36<br>D)28<br>E)8
Q140: The internal energy can be increased by
Q157: Which one of the following orbitals can