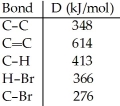
Using the table of bond dissociation energies,the ΔH for the reverse of following gas-phase reaction is ________ kJ. 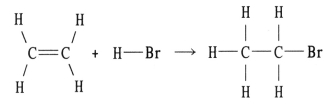
Definitions:
Institutions
Organizations or structures that are established for a particular purpose, often involved in public service, finance, or education.
Capital Market Instrument
Financial securities used to raise capital in public and private markets, including stocks and bonds.
Treasury Bond
A Treasury Bond is a fixed-interest government debt security with a maturity of more than ten years.
Common Stock
Equity ownership in a corporation, with voting rights and a share in dividends.
Q36: The value of ΔH° for the reaction
Q45: Which equation correctly represents the electron affinity
Q61: How many equivalent resonance forms can be
Q88: The main component of air is oxygen.
Q90: Which of the following elements is the
Q91: A gas originally at 27 °C and
Q97: _ is isoelectronic with helium.<br>A)H<sup>-</sup><br>B)H<sup>+</sup><br>C)H<br>D)B<sup>3-</sup><br>E)N<sup>3-</sup>
Q98: Of the choices below,which gives the order
Q136: In a C=C bond,the σ bond results
Q175: Lithium ion salts were originally found in