Using the table of bond dissociation energies,the ΔH for the following reaction is ________ kJ. 2HCl (g) + F2 (g) → 2HF (g) + Cl2 (g) 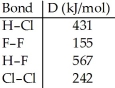
Definitions:
Basis Points
A financial measurement unit representing the percentage shift in the value or rate of a financial asset, which is equal to one hundredth of a percent.
Yield To Maturity
The total return anticipated on a bond if the bond is held until its maturity date, expressed as an annual rate.
Duration
A measure of the sensitivity of a bond's or fixed income portfolio's price to changes in interest rates, often used to manage interest rate risk.
Modified Duration
A measure of the sensitivity of a bond's price to changes in interest rates, specifically reflecting how much the duration changes for a 1% change in yield.
Q3: Using the table of average bond energies
Q16: An antibonding MO _ the corresponding bonding
Q41: A transition in the Bohr hydrogen atom
Q72: A valid Lewis structure of _ cannot
Q79: What is the wavelength (nm)of light emitted
Q84: All of the orbitals in a given
Q128: Coal contains hydrocarbons of high molecular weight
Q140: How many valence electrons are in the
Q142: The n = 1 shell contains _
Q143: Based on the octet rule,magnesium most likely