Using the table of bond dissociation energies,the ΔH for the following reaction is ________ kJ. 2HCl (g) + F2 (g) → 2HF (g) + Cl2 (g) 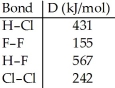
Definitions:
Direct Materials
Materials directly related and identifiable in the production of a particular item.
Finished Goods
Products that are finished being made but haven't been purchased by consumers yet.
Principal Accounting Record
The primary document or ledger that records a company's financial transactions and balances, often referred to as the General Ledger.
Job Cost Sheet
A financial record used in cost accounting to track the expenses associated with a specific job or project, aiding in budgeting and profitability analysis.
Q1: Two gases start to escape from a
Q32: The Lewis structure of PF<sub>3</sub> shows that
Q33: What is the de Broglie wavelength (m)of
Q41: As the number of covalent bonds between
Q75: A sample of He gas (2.35 mol)occupies
Q75: In comparing the same two atoms bonded
Q87: Peroxide or superoxides can be formed with
Q103: Based on molecular orbital theory,the bond order
Q106: Of the hydrogen halides,only _ is a
Q130: The ΔH<sub>rxn </sub>for the combustion of methane