A gas vessel is attached to an open-end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below. 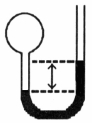 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
Definitions:
Q25: What is the molality of a 36.1%
Q88: The intermolecular force(s)responsible for the fact that
Q92: An aqueous solution with a concentration of
Q103: Write a balanced equation for the reaction
Q116: The phase diagram of a substance is
Q116: Which alkaline earth metal will not react
Q121: Which one of the following substances will
Q128: What is the hybridization of the I
Q135: There is/are _ π bond(s)in the molecule
Q168: How many molecules are there in 4.00