Using the van der Waals equation,the pressure in a 22.4 L vessel containing 1.00 mol of neon gas at 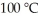 is ________ atm. (a = 0.211 L2-atm/mol2,b = 0.0171 L/mol)
is ________ atm. (a = 0.211 L2-atm/mol2,b = 0.0171 L/mol)
Definitions:
Marginal Utility
Additional contentment or usefulness achieved through the use of one more unit of a good or service.
Utility
In the field of economics, the complete pleasure derived from the consumption of a product or service.
Budget
A projection of revenues and expenses for a specified timeframe.
Total Utility
The total satisfaction or benefit received by consuming a particular amount of a good or service.
Q24: A reaction was found to be zero
Q54: The phrase "like dissolves like" refers to
Q60: All of the following are natural polymers
Q65: The formal charge on nitrogen in NO<sub>3</sub><sup>-</sup>
Q78: If 3.21 mol of a gas occupies
Q96: What type(s)of intermolecular forces exist between Cl<sub>2</sub>
Q96: Two deviations of real gases from ideal
Q107: There can be two equivalent best resonance
Q148: What is the vapor pressure (mm Hg)of
Q163: The electron-domain geometry of the AsF<sub>6</sub><sup>-</sup> ion