A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 751 torr.Use Boyle's law to calculate the pressure (torr) when the volume is reduced to 7.25 L at a constant temperature of 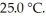
Definitions:
External Environments
Factors outside an organization that can impact its performance, such as economic conditions, competition, and regulatory changes.
Environmental Practices
Methods or processes employed by individuals, businesses, or governments aimed at reducing their impact on the natural environment.
Mobile Phones
Portable electronic devices used for communication, including voice calls, text messaging, and Internet access, integral to modern connectivity.
HR Services
A range of services provided by the Human Resources department, including recruitment, training, and employee relations.
Q11: The pressure exerted by 1.0 mol of
Q13: Define a body-centered cubic lattice.
Q24: Which of the following is not part
Q33: Which produces the greatest number of ions
Q34: Electrons in core orbitals contribute to atom
Q68: The dissolution of water in octane (C<sub>8</sub>H<sub>18</sub>)is
Q75: A solution is prepared by dissolving 15.0
Q75: A sample of He gas (2.35 mol)occupies
Q77: According to molecular orbital theory,the bond order
Q130: A 81.5 g sample of calcium chloride