Zinc reacts with aqueous sulfuric acid to form hydrogen gas: Zn (s) + 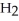
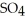 (aq) → Zn
(aq) → Zn 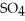 (aq) +
(aq) + 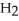 (g)
(g)
In an experiment,177 mL of wet 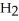 is collected over water at 27 °C and a barometric pressure of
is collected over water at 27 °C and a barometric pressure of 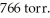 The vapor pressure of water at 27 °C is 26.74 torr.The partial pressure of hydrogen in this experiment is ________ atm.
The vapor pressure of water at 27 °C is 26.74 torr.The partial pressure of hydrogen in this experiment is ________ atm.
Definitions:
Electrolyte Imbalance
A condition where the levels of electrolytes (minerals like sodium, potassium, and calcium) in the body are either too high or too low, affecting bodily functions.
Thiazide Diuretics
A class of medications that promotes the removal of salt and water from the body, mainly used to treat high blood pressure.
Osteoporosis
A bone disease characterized by a decrease in bone mass and density, leading to an increased risk of fractures.
Steroids
A class of biologically active compounds with various medical uses, including reducing inflammation and treating autoimmune diseases.
Q34: The average rate disappearance of A between
Q49: When argon is placed in a container
Q50: Which energy change corresponds to the first
Q63: What is the molality of a 10.0%
Q67: What is the mole fraction of hydrochloric
Q88: How many equivalent resonance structures can be
Q90: The F-B-F bond angle in the BF<sub>3</sub>
Q122: Which one of the following concentration units
Q131: When solutions of strong electrolytes in water
Q137: The density of air at STP is