Calculate the enthalpy change (in kJ) associated with the conversion of 25.0 grams of ice at -4.00 °C to water vapor at 109.0 °C.The specific heats of ice,water,and steam are 2.09 J/g-K,4.18 J/g-K,and 1.84 J/g-K,respectively.For H2O,Δ 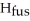 = 6.01 kJ/mol and Δ
= 6.01 kJ/mol and Δ 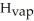 = 40.67 kJ/mol.
= 40.67 kJ/mol.
Definitions:
Interval Estimate
An estimate of a population parameter that provides a range of values believed to contain the parameter with a certain level of confidence.
Population Mean
The average of a set of values, taken from the entire population of data.
Confidence Level
The probability that a population parameter will fall between a set of values for a certain proportion of times.
Confidence Interval
A range of values, derived from sample data, that is likely to contain the value of an unknown population parameter with a specified level of confidence.
Q19: The total number of π bonds in
Q41: The pressure of a sample of CH<sub>4</sub>
Q47: Crystalline solids differ from amorphous solids in
Q50: The primary source of the specificity of
Q85: What is the osmotic pressure (in atm)of
Q90: Which one of the following gases would
Q100: How many moles of B are present
Q129: What is the % by mass of
Q138: What is the solubility concentration (M)of nitrogen
Q139: How many liters of O<sub>2</sub> are consumed