Ethanol ( 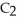
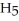 OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -60 °C?
OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ/mol.The specific heats of solid and liquid ethanol are 0.97 J/g-K and 2.3 J/g-K,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -60 °C?
Definitions:
Systematic Clinical Assessments
A structured process of evaluating the nature and severity of psychological disorders through standardized tests and methods.
Economic Pressures
Financial stressors that influence individuals' or entities' decisions due to constraints on resources.
Combined Treatment
An approach to therapy that uses more than one type of treatment, such as medication and psychotherapy, simultaneously.
Psychiatrist
A physician who in addition to medical school has completed three to four years of residency training in the treatment of abnormal mental functioning.
Q5: According to the phase diagram shown above,what
Q35: The equilibrium constant for the gas phase
Q41: Consider the following chemical reaction: CO (g)+
Q75: Of the following substances,_ has the highest
Q90: Which one of the following gases would
Q109: The pressure exerted by a column of
Q110: The H-B-H bond angle in BH<sub>3</sub> is
Q113: The hybridizations of iodine in IF<sub>3 </sub>and
Q128: What is the hybridization of the I
Q165: The molecular geometry of the H<sub>3</sub>O<sup>+</sup> ion