Calculate the vapor pressure of a solution made by dissolving 109 grams of glucose (molar mass = 180.2 g/mol) in 920.0 ml of water at 25 °C.The vapor pressure of pure water at 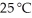 is
is 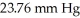 .Assume the density of the solution is 1.00 g/ml.
.Assume the density of the solution is 1.00 g/ml.
Definitions:
Receivables Balance
The total amount of money owed to a company by its customers for goods or services delivered or used but not yet paid for.
Accounts Receivable Turnover
This ratio measures how efficiently a company collects revenue from its customers by dividing total sales by the average accounts receivable during a specific period.
Credit Sales
Sales transactions where the payment is deferred to a future date, essentially extending credit to the purchaser.
Credit Period
The amount of time allowed by a seller for a buyer to pay for goods or services received, typically expressed in days.
Q7: An unknown metal crystallizes in a body-centered
Q26: Which one of the following is a
Q39: Pure solids and pure _ are excluded
Q50: Given the following reaction at equilibrium,if K<sub>p</sub>
Q61: 0.7515 moles of nitrogen gas and 0.1135
Q69: What is the chemical formula for the
Q71: Given the following reaction at equilibrium at
Q87: Consider the following reaction: 3A → 2B<br>The
Q91: Which solution has the greatest buffering capacity?<br>A)0.335
Q130: A 81.5 g sample of calcium chloride