A solution containing 10.0 g of an unknown liquid and 90.0 g water has a freezing point of -3.33 °C.Given 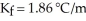 for water,the molar mass of the unknown liquid is
for water,the molar mass of the unknown liquid is 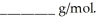
Definitions:
Nuclear Chain Reaction
A self-sustaining series of nuclear fissions in which the release of neutrons in one reaction induces at least one other nuclear reaction.
The Manhattan Project
A research and development project during World War II that produced the first nuclear weapons, led by the United States with the support of the UK and Canada.
War Production Board
An agency of the United States government during World War II tasked with overseeing the production and allocation of materials and fuel necessary for military operations.
Civilian to Military Production
The transition of manufacturing and production processes from civilian to military goods, typically during periods of war or military buildup.
Q45: Which of the following salts will produce
Q49: All of the following are polymers except
Q52: Of the following,which is the strongest acid?<br>A)HIO<sub>4</sub><br>B)HIO<sub>3</sub><br>C)HIO<sub>2</sub><br>D)HIO<br>E)The
Q55: A reaction vessel is charged with hydrogen
Q59: Which of the following expressions is the
Q75: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt=" = 0.0198 at
Q107: The rate of disappearance of HBr in
Q123: In the reaction<br>BF<sub>3</sub> + F<sup>-</sup> → BF<sub>4</sub><sup>-</sup><br>BF<sub>3</sub>
Q136: Of the following,_ is a weak acid.<br>A)HF<br>B)HCl<br>C)HBr<br>D)HNO<sub>3</sub><br>E)HClO<sub>4</sub>
Q180: Mixing one s atomic orbital and one