A mixture containing 33.0 g of an unknown liquid and 230.0 g of water has a freezing point of -1.12 °C.Given 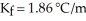 for water,what is the molar mass (g/mol) of the unknown liquid?
for water,what is the molar mass (g/mol) of the unknown liquid?
Definitions:
Vocational Degree
A qualification awarded for the completion of education or training directly related to a specific trade or profession.
High School Degree
A certificate or diploma awarded upon successfully completing the required course of studies in a secondary school or high school.
College Degree
A degree conferred by higher education institutions to students who have shown proficiency in a particular area of study or professional field after finishing a prescribed program.
Deindustrialization
A social and economic change resulting from the reduction of industrial activity, especially manufacturing.
Q1: A gas is _ and assumes _
Q26: In a solution,when the concentrations of a
Q34: The average rate disappearance of A between
Q36: Which monomer is polymerized to make natural
Q68: How many liters of CO<sub>2</sub> are formed
Q82: Which reaction will shift to the right
Q86: The decomposition of [A] in solution at
Q94: Which of the following molecules has hydrogen
Q116: Which of the following could be added
Q152: Calcium hydride ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1194/.jpg" alt="Calcium hydride