A mixture containing 41.0 g of an unknown nonelectrolyte and 160.0 g of water has a freezing point of -1.34 °C.Given 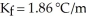 for water,what is the molecular weight (g/mol) of the unknown solute?
for water,what is the molecular weight (g/mol) of the unknown solute?
Definitions:
Talk Negatively
Engaging in conversation that focuses on criticism, pessimism, or highlighting flaws without constructive purpose.
Exit Statement
A formal declaration made when leaving a position or situation, sometimes including reasons for the departure.
Perception
The process of interpreting, analyzing, and assigning meaning to sensory information.
Perceptual Process
The sequence of steps that involves receiving, organizing, and interpreting sensory information to understand the environment.
Q2: The conjugate base of HSO<sub>4</sub><sup>-</sup> is _.<br>A)H<sub>2</sub>SO<sub>4</sub><br>B)HSO<sub>4</sub><sup>+</sup><br>C)H<sup>+</sup><br>D)SO<sub>4</sub><sup>2-</sup><br>E)HSO<sub>3</sub><sup>+</sup>
Q9: The properties of graphene include _.<br>A)large thermal
Q10: What fraction of the volume of each
Q17: What is the percent gold in a
Q27: Decreasing the pH of blood will cause
Q57: What is the predominant intermolecular force in
Q103: In which of the following aqueous solutions
Q113: Hydrogen bonding is a special case of
Q119: What is the concentration (in M)of hydronium
Q130: Define a heterogeneous catalyst.