Multiple Choice
Determine the freezing point (°C) of a 0.015 molal aqueous solution of MgSO4.Assume i = 2.0 for MgSO4.The molal freezing-point-depression constant of water is 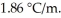
Definitions:
Related Questions
Q17: A saturated solution _.<br>A)contains dissolved solute in
Q29: Classify the following compounds as weak bases
Q31: The value of K<sub>eq</sub> for the equilibrium
Q37: Heats of vaporization are greater than heats
Q41: Which of the following could be added
Q43: Define Beer's Law.
Q56: The relationship between the concentrations of reactants
Q140: A sample of hydrogen gas (3.2 L)at
Q141: What is the molality of a 35.0%
Q167: Zinc reacts with aqueous sulfuric acid to