A solution is prepared by dissolving 2.00 g of glycerin ( 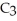
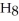
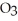 ) in 201 g of ethanol
) in 201 g of ethanol 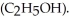 The freezing point of the solution is ________°C.The freezing point of pure ethanol is
The freezing point of the solution is ________°C.The freezing point of pure ethanol is 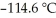 at 1 atm.The molal-freezing-point-depression constant (
at 1 atm.The molal-freezing-point-depression constant ( 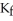 ) for ethanol is
) for ethanol is 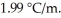 The molar masses of glycerin and of ethanol are 92.1 g/mol and 46.1 g/mol,respectively.
The molar masses of glycerin and of ethanol are 92.1 g/mol and 46.1 g/mol,respectively.
Definitions:
Internal Locus Of Control
A psychological concept referring to individuals who believe they have control over the events in their lives, as opposed to external forces or chance.
Self-Confidence
The belief in one's own abilities and judgment, often linked to success and positive outcomes in tasks.
Narcissism
A personality trait characterized by an inflated sense of self-importance, a deep need for admiration, and a lack of empathy for others.
Socialized Need
A desire or motivation that has been influenced by social factors and the individual's interaction with their social environment.
Q21: A 0.10 M aqueous solution of the
Q21: After swimming in the ocean for several
Q21: The rate constant for a second-order reaction
Q34: If a reaction is exothermic,_ the reaction
Q64: The overall reaction order is the sum
Q67: The equilibrium constant for the gas phase
Q68: The dissolution of water in octane (C<sub>8</sub>H<sub>18</sub>)is
Q76: The conjugate base of NH<sub>3</sub> is _.<br>A)NH<sub>2</sub><sup>-</sup><br>B)NH<sub>4</sub><sup>+</sup><br>C)NH<sub>2</sub>OH<br>D)H<sub>3</sub>O<sup>+</sup><br>E)OH<sup>-</sup>
Q80: The overall reaction below is _ and
Q84: A unimolecular elementary reaction involves _ reactant