Calculate the freezing point of a solution containing 20 grams of KCl and 2200.0 grams of water.The molal-freezing-point-depression constant ( 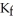 ) for water is
) for water is 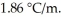 (Assume 100% ionization of KCl.)
(Assume 100% ionization of KCl.)
Definitions:
Acknowledging Desirable Behavior
involves recognizing and reinforcing positive actions or conduct, particularly as a strategy in behavior modification.
Contextual Factors
Conditions or circumstances that exist outside of the individual but can significantly influence both behavior and development.
Differences in Status and Power
Variations in the hierarchy and authority levels among individuals or groups within a social structure or organization.
History of the Relationship
The chronological record of interactions, contributions, and significant events impacting the dynamics between individuals or groups.
Q30: What is the conjugate acid of NH<sub>2</sub><sup>-</sup>?<br>A)NH<sub>2</sub><sup>+</sup><br>B)NH<sub>3</sub><sup>+</sup><br>C)NH<sub>4</sub><sup>+</sup><br>D)NH<sub>3</sub><br>E)NH<sub>4</sub>OH
Q38: The pH of a solution prepared by
Q43: Of the following,_ is an exothermic process.<br>A)melting<br>B)subliming<br>C)freezing<br>D)boiling<br>E)All
Q65: A sample of gas (1.9 mol)is in
Q81: The K<sub>eq</sub> for the equilibrium below is
Q90: In which of the following aqueous solutions
Q92: What change will be caused by addition
Q103: A solution of NaF is added dropwise
Q131: The rate-determining step is the _ elementary
Q171: A mixture of Xe,Kr,and Ar has a