Multiple Choice
Calculate the vapor pressure of a solution made by dissolving 109 grams of glucose (molar mass = 180.2 g/mol) in 920.0 ml of water at 25 °C.The vapor pressure of pure water at 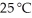 is
is 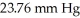 .Assume the density of the solution is 1.00 g/ml.
.Assume the density of the solution is 1.00 g/ml.
Identify factors leading to a loss of intellectual capital and their impacts on organizations.
Recognize and differentiate between organizational citizenship behaviors and counterproductive work behaviors.
Understand the role and significance of corporate social responsibility within organizations.
Comprehend the concept and components of High-Performance Work Practices (HPWP) and their relevance in leveraging human capital.
Definitions:
Related Questions
Q13: The K<sub>a</sub> of some acid,HA,at 25.0 °C
Q18: _ generally differ from compounds in that
Q20: Arrange the following gases in order of
Q52: Which species has London dispersion forces as
Q56: A substance that is capable of acting
Q71: Real gases do not behave ideally at
Q73: Which one of the following is the
Q78: On a phase diagram,the critical temperature is
Q80: In the _ liquid-crystalline phase,the molecules are
Q85: If the rate law for the reaction